Lemon Power
Learning Objectives
Electricity is a form of energy that students encounter every day. Students will build their own circuit with basic materials, using a lemon or a potato to act as the battery, to get hands-on experience with generating electricity.
Associated Curriculum Topic
Electricity and Electrical Devices
Materials
per group of students
- electrical leads with alligator clips
- at least 4 lemons or potatoes
- one low-current, light emitting diode (LED) clock requiring approximately 1.5 volts (or a small LED bulb) (available at Radio Shack or other electronics shops or websites)
- one piece of zinc for each lemon or potato (galvanized nails work well)
- one piece of copper for each lemon or potato (pennies from before 1996, or small pieces of copper pipe work better – available at Home Depot among other places)
- Multi-meter (if you can get access to one – can be shared among all groups)
Introduction/Motivation
In this experiment students will construct their very own battery from either potatoes or lemons. Both lemons and potatoes contain acids: the lemon is mostly made up of citric acid (C6H8O7) while the potato contains a mild phosphoric acid (H3PO4). These acids allow both materials to act like a battery, which is commonly referred to as a voltaic battery. The positive and negative terminals are easily produced by simply placing two different metals into either of the two materials. These two metals are referred to as electrodes. In this experiment there are two types of electrodes, the cathode, which gains electrons and the anode, which releases electrons. The two most commonly used metals are zinc and copper, since zinc is a good source of electrons (acts as an anode) and copper likes to collect as many electrons as possible (acts like a cathode). By connecting a wire between the two electrodes, electrons in the acidic material are free to flow from the zinc towards the copper electrodes and create a current as well as voltage difference. Surprisingly a potato can produce approximately one volt while a lemon can produce approximately 0.7 volts. In order to light up an LED students will need to connect about 3-4 potatoes or lemons in series (one after the other).
Procedure
The following procedure is the same for both the lemons and potatoes:
- For best results, roll the lemons on the table pressing downward to squish them a bit. (Try not to break the skin of the lemon in this step). You do not need to do this step with the potatoes.Press a piece of copper into each of the potatoes or lemons.
- Press a galvanized nail into each of the potatoes or lemons. Try to get the piece of copper and zinc as close as possible without touching.
- Attach one end of an alligator clip to one of the copper pieces. Attach the other end of the alligator clip to a galvanized nail of a different lemon or potatoes. Repeat this until you have an alligator clip on each of the copper pieces and zinc pieces.
- Detach one of the alligator clips from one of the galvanized nails and attach it to the positive lead of a low current LED (typically this is the longer of the two leads).
- Attach a new alligator clip to the galvanized nail, which you just removed the alligator clip from. Using the alligator clip on the other end of the wire connect it to the negative terminal of the LED (typically shorter).
- The LED should light up upon completing the circuit. See the figure on the following page for guidance.
Trouble shooting:
- First try reversing the connections on the LED bulb.
- With the multi-meter if you have access to one, check the voltage. If you do not have enough voltage, you may want to add another lemon or potato.
- The copper pennies may have oxidized since the date they were released and you may need to lightly sand them to remove the oxidation layer. Pennies since 1996 are a zinc copper blend and will not work as well.
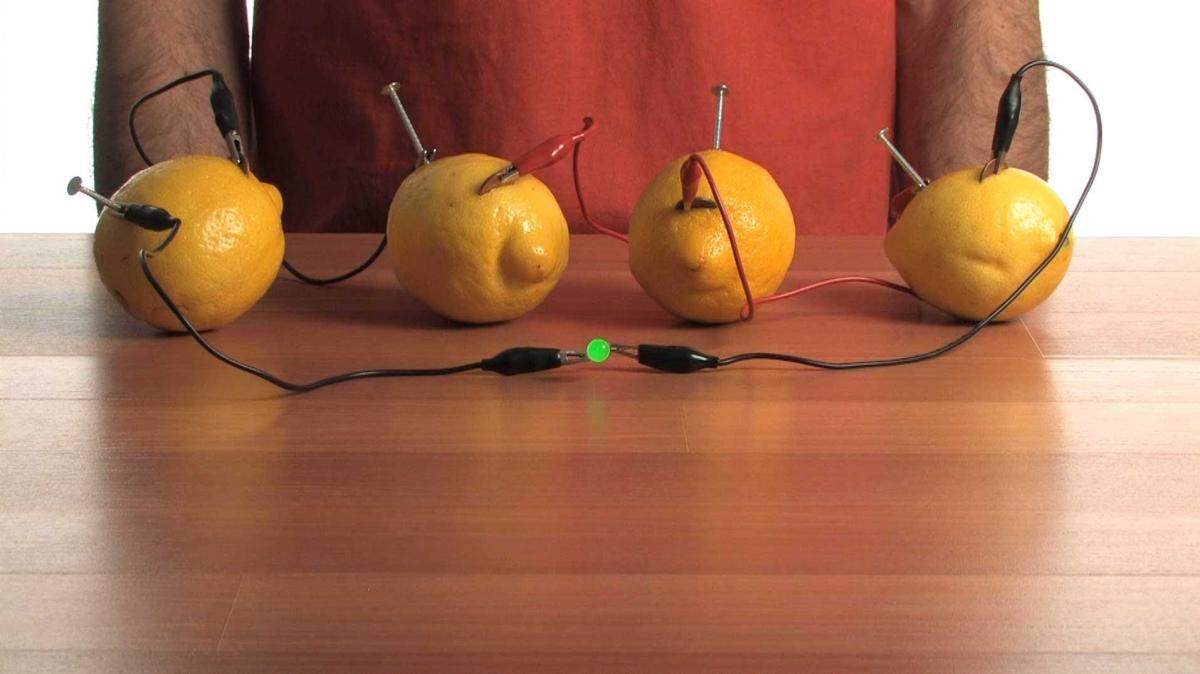
Image from http://send104b.com/av/d31968u1/battery-sick-power/1186so9/
Investigating Questions
- What is an electron?
- What is an acid?
- What is an electrode?
- What is voltage/current?
- Do you think other metals would work instead of copper and zinc?
Adapted from Teach Engineering